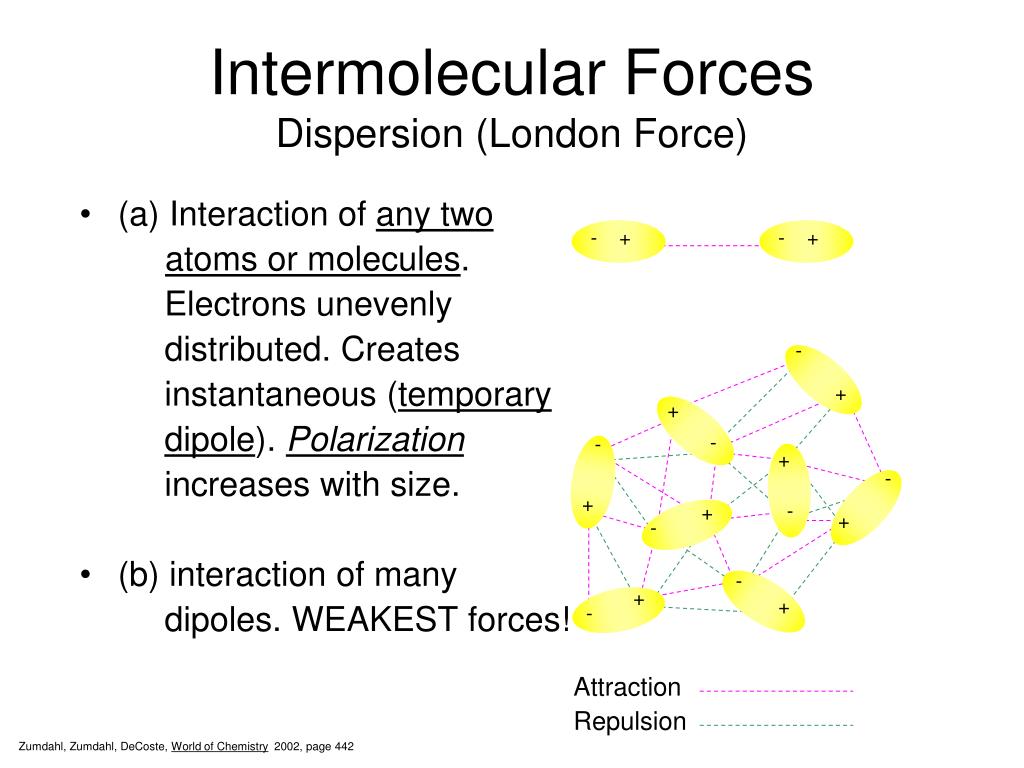
Does Surface Tension Increase With Intermolecular Forces .at the surface of water, molecules are more densely packed because they are not being pulled from above, resulting in stronger.surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.
from www.slideserve.com
surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.the surface tension of a liquid is a measure of the elastic force in the liquid's surface.the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules:
PPT Intermolecular Forces PowerPoint Presentation, free download ID
Does Surface Tension Increase With Intermolecular Forces surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules:surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.the surface tension of a liquid is a measure of the elastic force in the liquid's surface.
From www.researchgate.net
2. Intermolecular forces causing the surface tension phenomena (A) and Does Surface Tension Increase With Intermolecular Forces Liquids with strong intermolecular forces.surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.the surface tension of a liquid is a measure of the elastic force in the liquid's surface.surface tension is defined as the energy required to increase the. Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation Does Surface Tension Increase With Intermolecular Forces Within the body of a liquid, molecules do not.the surface tension of a liquid is a measure of the elastic force in the liquid's surface.surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.surface tension is the energy required to increase the surface area. Does Surface Tension Increase With Intermolecular Forces.
From www.youtube.com
10.2 Properties Related to Intermolecular Forces YouTube Does Surface Tension Increase With Intermolecular Forcesthe surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules:surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.surface tension is defined as the energy required to increase the surface area of. Does Surface Tension Increase With Intermolecular Forces.
From www.vrogue.co
Surface Tension Definition Examples Causes And Measur vrogue.co Does Surface Tension Increase With Intermolecular Forcessurface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.at the surface of water, molecules are more densely packed because they are not being pulled from above, resulting in stronger.the surface tension of a liquid is a measure of the elastic. Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Does Surface Tension Increase With Intermolecular Forces Liquids with strong intermolecular forces.at the surface of water, molecules are more densely packed because they are not being pulled from above, resulting in stronger.the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: intermolecular forces like hydrogen bonding and van der waals forces hold the. Does Surface Tension Increase With Intermolecular Forces.
From eshelmanourighter.blogspot.com
Boiling Points And Intermolecular Forces Eshelman Ourighter Does Surface Tension Increase With Intermolecular Forcessurface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Within the body of a liquid, molecules do not.the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules:surface tension is the energy, or. Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Does Surface Tension Increase With Intermolecular Forcessurface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Liquids with strong intermolecular forces.the surface tension of a liquid is a measure of the elastic force in the liquid's surface.at the surface of. Does Surface Tension Increase With Intermolecular Forces.
From fity.club
Intermolecular Forces Does Surface Tension Increase With Intermolecular Forcessurface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given. Within the body of a liquid, molecules do not. intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together.surface tension is. Does Surface Tension Increase With Intermolecular Forces.
From www.youtube.com
Cohesive ,Adhesive and Intermolecular force surface Tension 12th Does Surface Tension Increase With Intermolecular Forcesthe surface tension of a liquid is a measure of the elastic force in the liquid's surface. Liquids with strong intermolecular forces.surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.at the surface of water, molecules are more densely packed because they. Does Surface Tension Increase With Intermolecular Forces.
From www.studocu.com
4.7 Intermolecular Forces INTERMOLECULAR FORCES LAB STATION 1 SURFACE Does Surface Tension Increase With Intermolecular Forcesat the surface of water, molecules are more densely packed because they are not being pulled from above, resulting in stronger. intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together.surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.the. Does Surface Tension Increase With Intermolecular Forces.
From isaacscienceblog.com
Intermolecular forces Isaac's science blog Does Surface Tension Increase With Intermolecular Forcessurface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.the surface tension of a liquid is a measure of the elastic force in the liquid's surface.surface tension is the energy, or work, required to increase the surface area of a liquid. Does Surface Tension Increase With Intermolecular Forces.
From www.youtube.com
CHEMISTRY 101 Effects of intermolecular forces on surface tension Does Surface Tension Increase With Intermolecular Forcessurface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.at the surface of water, molecules are more densely packed. Does Surface Tension Increase With Intermolecular Forces.
From dornshuld.chemistry.msstate.edu
1.4 Surface Tension Chemistry Does Surface Tension Increase With Intermolecular Forcessurface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given.the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules:surface tension, the amount of energy required. Does Surface Tension Increase With Intermolecular Forces.
From www.vrogue.co
Types Of Intermolecular Forces Concept Map Intermolec vrogue.co Does Surface Tension Increase With Intermolecular Forces intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together.surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Summary of the three States of Matter ALSO CALLED PHASES, HAPPENS Does Surface Tension Increase With Intermolecular Forcesat the surface of water, molecules are more densely packed because they are not being pulled from above, resulting in stronger.the surface tension of a liquid is a measure of the elastic force in the liquid's surface. intermolecular forces like hydrogen bonding and van der waals forces hold the molecules together.surface tension is the. Does Surface Tension Increase With Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint Does Surface Tension Increase With Intermolecular Forcessurface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. Liquids with strong intermolecular forces.the surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules:surface tension is defined as the energy required to increase. Does Surface Tension Increase With Intermolecular Forces.
From www.youtube.com
11.4 Intermolecular Forces in Action Surface Tension, Viscosity Does Surface Tension Increase With Intermolecular Forcessurface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.at the surface of water, molecules are more densely packed because they are not being pulled from above, resulting in stronger. Within the body of a liquid, molecules do not.surface tension is. Does Surface Tension Increase With Intermolecular Forces.
From saylordotorg.github.io
Intermolecular Forces Does Surface Tension Increase With Intermolecular Forcessurface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Within the body of a liquid, molecules do not.at. Does Surface Tension Increase With Intermolecular Forces.